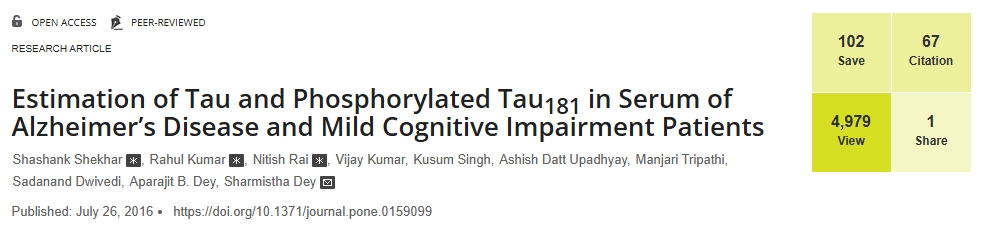
Neurons are set apart from other cell types by distinct features. These include post-mitotic status requiring their extended survival, intricate morphology defining their participation in complex neural circuits and dynamic regulation of individual synapses. These distinct features necessitate specialized mechanisms to respond to stress and restore homeostasis to promote neuronal survival. I used Drosophila photoreceptors as my model and ambient constant light paradigm (LL) as stressor to understand the mechanisms regulating neuronal structural plasticity. I discovered that, under LL photoreceptor neurons reduce their size by almost one-third. This adaptive change requires multiple stress response pathways and is reversible upon removal of the stressor.

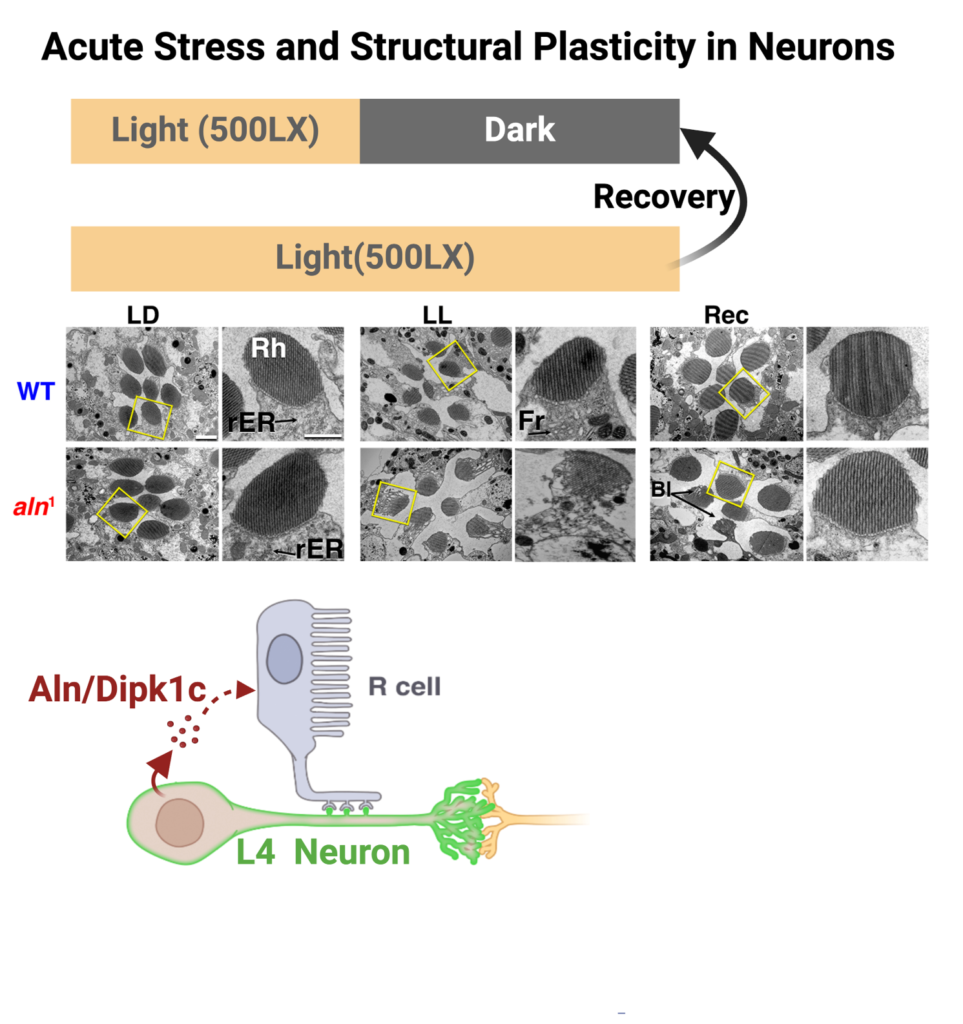
Sensory disabilities have been identified as significant risk factors for dementia but underlying molecular mechanisms are unknown. A common aspect of different neurodegenerative disorders, including dementia, is dysregulated protein homeostasis or proteostasis. I investigated the impact of failure of sensory output in different models of drosophila and mouse on brain-wide proteostasis and found that sensory disabilities disrupt proteostasis in both Drosophila and mouse models.

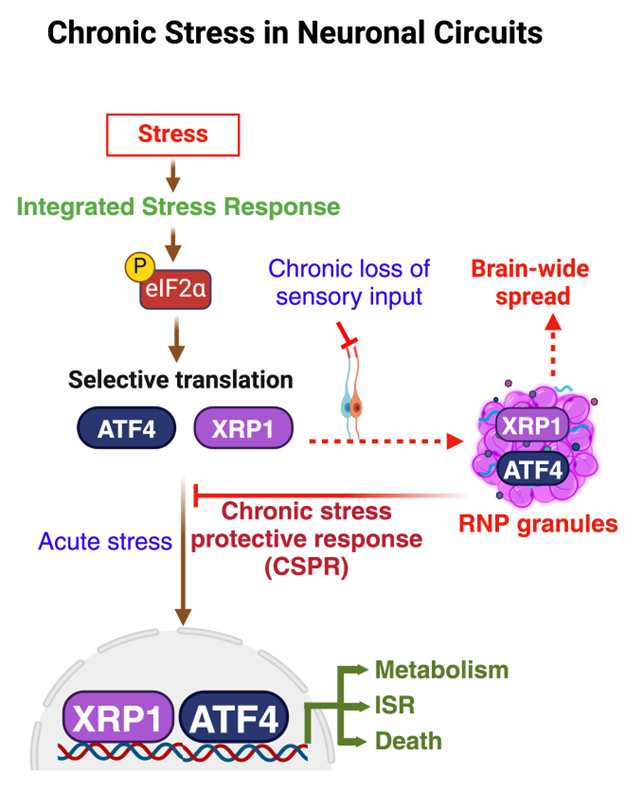
During my doctoral research I found that the level of Tau, p-Tau181, and 5-Lipoxygenase in serum of Alzheimer’s and MCI patients is elevated. This was the first report for a blood-based biomarker in the field. I used Surface Plasmon Resonance technology to measure serum levels of these proteins. Concretely, I designed and synthesized phospho-peptide epitope of p-Tau181 to quantify the serum level. As blood-based markers have several advantages over the invasive CSF or expensive MRI and PET-Scans, my research established a foundation for less invasive and highly cost-effective circulatory biomarkers for Alzheimer’s disease.